Our results might also be explained if the female-sized
BSTc in the transsexual group was due to the lack of androgens, because
they had all been orchidectomized except for T4. We therefore studied two
other men who had been orchidectomized because of cancer of the prostate
(one and three months before death: S4 and S3, respectively), and found
that their BSTc sizes were at the high end of the normal male range. The
BSTc size of the single transsexual who had not been orchidectomized (T4)
ranged in the middle of the transsexual scores (Fig. 3). Not only were
five of the transsexuals orchidectomized, they all used the antiandrogen
cyproterone acetate (CPA). A CPA effect on the BSTc does not seem likely,
because T6 had not taken CPA for the past 10 years, and T3 took no CPA
during the two years before death and still had a female-sized BSTc.
In summary, our observations suggest that the small size
of the BSTc in male-to-female transsexuals cannot be explained by differences
in adult sex hormone levels, but is established during development by an
organizing action of sex hormones, an idea supported by the fact that neonatal
gonadectomy of male rats and androgenization of the female rats indeed
induced significant changes in the number of neurons of the BST and suppressed
its sexual dimorphism [17,18].
Considered together with information from animals, then
our study supports the hypothesis that gender identity alterations may
develop as a result of an altered interaction between the development of
the brain and sex hormones [5,6]. The direct action of genetic factors
should also be considered on the basis of animal experiments [24].
We found no relationship between BSTc size and the sexual
orientation of transsexuals, that is, whether they were male-oriented (T1,T6),
female-oriented (T3,T2,T5), or both (T4). Furthermore, the size of the
BSTc of heterosexual men and homosexual men did not differ, which reinforced
the idea that the reduced BSTc size is independent of sexual orientation.
In addition, there was no difference in BSTc size between early-onset (T2,T5,T6)
and late-onset transsexuals (T1, T3), indicating that the decreased size
is related to the gender identity alteration per se rather than to the
age at which it becomes apparent. Interestingly, the very small BSTc in
transsexuals appears to be a very local brain difference. We failed to
observe similar changes in three other hypothalamic nuclei, namely, PVN,
SDN or SCN in the same individuals (unpublished data). This might be due
to the fact that these nuclei do not all develop at the same time, or to
a difference between these nuclei and the BST with respect to the presence
of sex hormone receptors or aromatase. We are now studying the distribution
of sex hormone receptors and the aromatase activity in various hypothalamic
nuclei in relation to sexual orientation and gender.
Acknowledgements
We thank Mr. B. Fisser, Mr. H. Stoffels, Mr. G. van der
Meulen, and Ms. T. Eikelboom and Ms. W.T.P. Verweij for their help, and
Drs. R.M. Buijs, M.A. Corner, E. Fliers, A. Walter and F.W. van Leeuwen
for their comments. Brain material was provided by the Netherlands Brain
Bank (coordinator Dr. R. Ravid). This study was supported by NWO.
References
Money, J. and Gaskin, Int. J. Psychiatry,
9 (1970/1971) 249.
Gooren, L.J.G., Psychoneuroencrinology, 15 (1990)
3-14.
Kawakami, M. and Kimura, F., Endocrinol. Jap.,
21 (1974) 125-130.
Emery, D.E. and Sachs, B.D., Physiol. Behav., 17
(1976) 803-806.
Editorials Lancet, 338 (1991) 603-604.
Swaab, D.F. and Hofman, M.A., TINS, 18 (1995) 264-270.
Money, J., Schwartz, M. and Lewis, V.G., Psychoneuroendocrinology,
9 (1984) 405- 414.
Sheridan, P.J., Endocrinology, 104 (1979) 130-136.
Commins, D. and Yahr, D., J. Comp. Neurol., 231
(1985) 473-489.
Jakab, R.L., Horvath, T.L., Leranth, C., Harada, N.
and Naftolin, F.J., Steroid Biochem. Molec. Biol., 44 (1993) 481-498.
Eiden, E.L., Hökfelt, T, Brownstein, M.J. and
Palkovits, M., Neuroscience, 15 (1985) 999-1013.
De Olmos, J.S. In: Paxinos, G. (Ed.), The Human
Nervous System, Academic Press, San Diego, 1990, pp. 597-710.
Woodhams, P.L., Roberts, G.W., Polak, J.M. and Crow,
T.J., Neuroscience, 8 (1983) 677-703.
Simerly, R.B., TINS, 13 (1990) 104-110.
Arluison, M., et al., Brain Res. Bull., 34 (1994)
319-337.
Bleier, R., Byne, W. and Siggelkow, I., J. Comp.
Neurol., 212 (1982) 118-130.
Del Abril, A., Segovia, S. and Guillamón, A.,
Dev. Brain Res., 32 (1987) 295-300.
Guillamón, A., Segovia, S. and Del Abril, A.,
Dev. Brain Res., 44 (1988) 281-290.
Allen, L.A. and Gorski, R.A., J. Comp. Neurol.,
302 (1990) 697-706.
Walter, A., Mai, J.K., Lanta, L. and Görcs, T.J.,
Chem. Neuroanat., 4 (1991) 281-298.
Claro, F., Segovia, S., Guilamón, A. and Del
Abril, A., Brain Res. Bull., 36 (1995) 1-10.
Simerly, R.B. and Swanson, L.W., Proc. Natl. Acad.
Sci. U.S.A., 84 (1987) 2087- 2091.
De Vries, G.J., J. Neuroendocrinol., 20 (1990)
1-13.
Pilgrim, Ch. and Reisert, I., Horm. metab. Res.,
24 (1992) 353-359.
Swaab, D.F., Zhou, J.N., Ehlhart, T. and Hofman, M.A.,
Brain Res., 79 (1994) 249- 259.
Zhou, J.N., Hofman, M.A. and Swaab, D.F., Neurobiol.
Aging (1995) in press.
Zhou, J.N., Hofman, M.A. and Swaab, D.F.,
Brain Res. 672 (1995) 285-288.
Swaab D.F. and Hofman M.A., Brain Res., 537 (1990)
141-148.
Correspondence and requests for materials to:
J.-N. Zhou, M.A. Hofman and D.F. Swaab
Graduate School Neurosciences Amsterdam
Netherlands Institute for Brain Research
Meibergdreef 33
1105 AZ Amsterdam ZO
The Netherlands
L.J.G. Gooren
Department of Endocrinology
Free University Hospital
1007 MB Amsterdam
The Netherlands
Email: lgooren@inter.nl.net
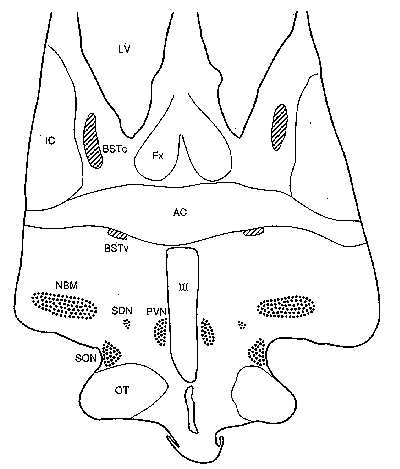
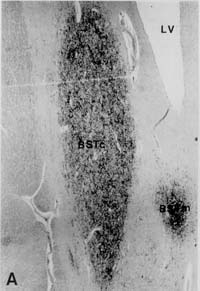

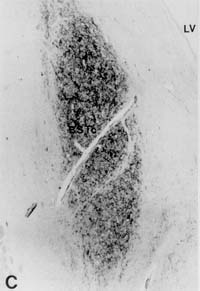
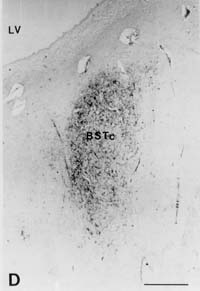
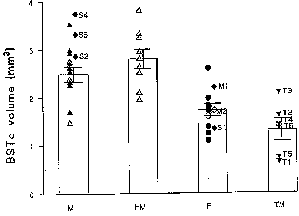 Figure 3: Volume of the BSTc innervated by VIP
fibres in presumed heterosexual males (M), homosexual males (HM), presumed
heterosexual females (F) and male-to-female transsexuals (TM). The six
transsexuals are numbered T1-T6. The patients with abnormal sex hormone
levels are numbered S1-S4. M1 and M2: postmenopausal women. Bars indicate
mean±SEM. Open symbols: individuals who died of AIDS. METHODS. Brains
of 42 subjects matched for age, postmortem time and duration of formalin
fixation were investigated. The autopsy was performed following the required
permission. For immunocytochemical staining of VIP, the paraffin sections
were hydrated and rinsed in TBS (Tris-buffered-saline: 0.05 M tris, 0.9%
NaCl, pH 7.6). The sections were incubated with 200 µl anti-VIP (Viper,
18/9/86) 1:1000 in 0.5% triton in TBS overnight at 4° C. The immunocytochemical
and morphometric procedures were performed as described extensively elsewhere
[25-27]. In brief, serial 6 m m sections of the BSTc were studied by means
of a digitizer (Calcomp 2000) connected to a HP-UX 9.0, using a Zeiss microscope
equipped with a 2.5x objective and with 10x (PLAN) oculars. Staining was
performed on every 50th section with anti-VIP. The rostral and caudal borders
of the BSTc were assessed by staining every 10th section in the area. The
volume of the BSTc was determined by integrating all the area measurements
of the BSTc sections that were innervated by VIP fibres. In a pilot study,
the size of the BSTc was measured on both sides in eight subjects (five
females and three males) and no left-right asymmetries were observed: the
left BSTc (1.71±0.16 mm3) was comparable in size to that
of the right BSTc (1.83±0.30 mm3) (P=0.79). No asymmetry
was observed in the BNST-dspm either [19]. The rest of our study was therefore
performed on one side of the brain only. Brain weight of the male transsexuals
(1385±75 g) was not different from that of the reference males (1453±25
g) (P=0.61) or that of the females (1256±35 g) (P=0.23). The cause
of death of the six transsexuals was suicide (T1), cardiovascular disease
(T2,T6), sarcoma (T3), AIDS, pneumonia, pericarditis (T4) and hepatitic
failure (T5). Sexual orientation of the subjects of the reference group
(12 men and 11 women) was generally not known, but presumably most of them
were heterosexual. Sexual orientation of nine homosexuals was registered
in the clinical records [28]. Differences among the groups were tested
two-tailed using the Mann-Whitney U test. A 5% level of significance was
used in all statistical tests.
Figure 3: Volume of the BSTc innervated by VIP
fibres in presumed heterosexual males (M), homosexual males (HM), presumed
heterosexual females (F) and male-to-female transsexuals (TM). The six
transsexuals are numbered T1-T6. The patients with abnormal sex hormone
levels are numbered S1-S4. M1 and M2: postmenopausal women. Bars indicate
mean±SEM. Open symbols: individuals who died of AIDS. METHODS. Brains
of 42 subjects matched for age, postmortem time and duration of formalin
fixation were investigated. The autopsy was performed following the required
permission. For immunocytochemical staining of VIP, the paraffin sections
were hydrated and rinsed in TBS (Tris-buffered-saline: 0.05 M tris, 0.9%
NaCl, pH 7.6). The sections were incubated with 200 µl anti-VIP (Viper,
18/9/86) 1:1000 in 0.5% triton in TBS overnight at 4° C. The immunocytochemical
and morphometric procedures were performed as described extensively elsewhere
[25-27]. In brief, serial 6 m m sections of the BSTc were studied by means
of a digitizer (Calcomp 2000) connected to a HP-UX 9.0, using a Zeiss microscope
equipped with a 2.5x objective and with 10x (PLAN) oculars. Staining was
performed on every 50th section with anti-VIP. The rostral and caudal borders
of the BSTc were assessed by staining every 10th section in the area. The
volume of the BSTc was determined by integrating all the area measurements
of the BSTc sections that were innervated by VIP fibres. In a pilot study,
the size of the BSTc was measured on both sides in eight subjects (five
females and three males) and no left-right asymmetries were observed: the
left BSTc (1.71±0.16 mm3) was comparable in size to that
of the right BSTc (1.83±0.30 mm3) (P=0.79). No asymmetry
was observed in the BNST-dspm either [19]. The rest of our study was therefore
performed on one side of the brain only. Brain weight of the male transsexuals
(1385±75 g) was not different from that of the reference males (1453±25
g) (P=0.61) or that of the females (1256±35 g) (P=0.23). The cause
of death of the six transsexuals was suicide (T1), cardiovascular disease
(T2,T6), sarcoma (T3), AIDS, pneumonia, pericarditis (T4) and hepatitic
failure (T5). Sexual orientation of the subjects of the reference group
(12 men and 11 women) was generally not known, but presumably most of them
were heterosexual. Sexual orientation of nine homosexuals was registered
in the clinical records [28]. Differences among the groups were tested
two-tailed using the Mann-Whitney U test. A 5% level of significance was
used in all statistical tests.